
It is often reported that Michelson got the idea from Kelvin, but there is little evidence to back this claim up.Īt the turn of the century, Kelvin wasn't saying that physics was finished. A similar statement was made twice by the German-American scientist Albert Michelson (1852–1931) as was discussed earlier in this book. This has been attributed to William Thomson, Lord Kelvin (1824–1907) in an address to the British Association for the Advancement of Science in 1900, but I haven't been able to find the primary source. All that remains is more and more precise measurement. There is nothing new to be discovered in physics now. Several people are reported to have said something like this


They agree with reality to a high degree of accuracy as tested in experiment after experiment.Īt the end of the 19th century, physics appeared to be at an apex. They are mathematically consistent in the sense that no one rule would ever violate another. They can be used to deliver spacecraft to the ends of the solar system with hyper-pinpoint accuracy. They were used to create the machines that launched two waves of industrial revolution - the first one powered by steam and the second one powered by electric current. They describe a universe consisting of bodies moving with clockwork predictability on a stage of absolute space and time. A relatively low-temperature object, such as a horseshoe forged by a blacksmith, appears red, whereas a higher-temperature object, such as the surface of the sun, appears yellow or white.Newton's laws of motion and universal gravitation, the laws of conservation of energy and momentum, the laws of thermodynamics, and Maxwell's equations for electricity and magnetism were all more or less nearly complete at the end of the 19th century. When heated, all objects emit electromagnetic radiation whose wavelength (and color) depends on the temperature of the object. As its temperature increases further it becomes yellow, white, and ultimately blue-white. When it becomes a little hotter, it appears dull red. Because the human eye cannot perceive light waves at lower frequencies, a black body, viewed in the dark at the lowest just faintly visible temperature, subjectively appears grey, even though its objective physical spectrum peaks in the infrared range. This emission is called blackbody radiation.Ī room temperature blackbody appears black, as most of the energy it radiates is infra-red and cannot be perceived by the human eye.
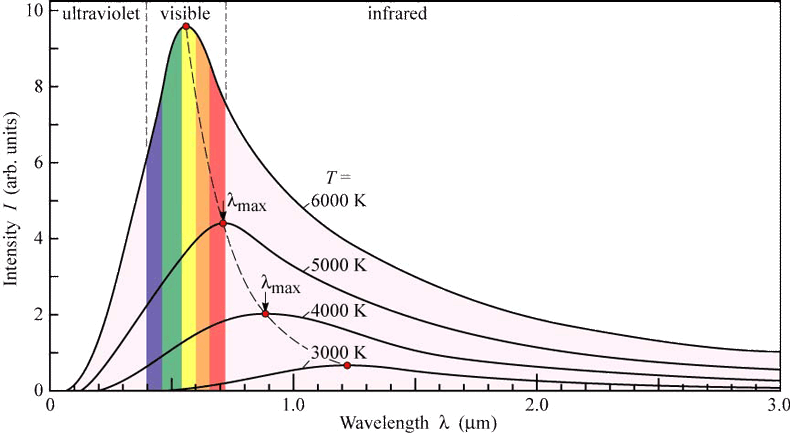
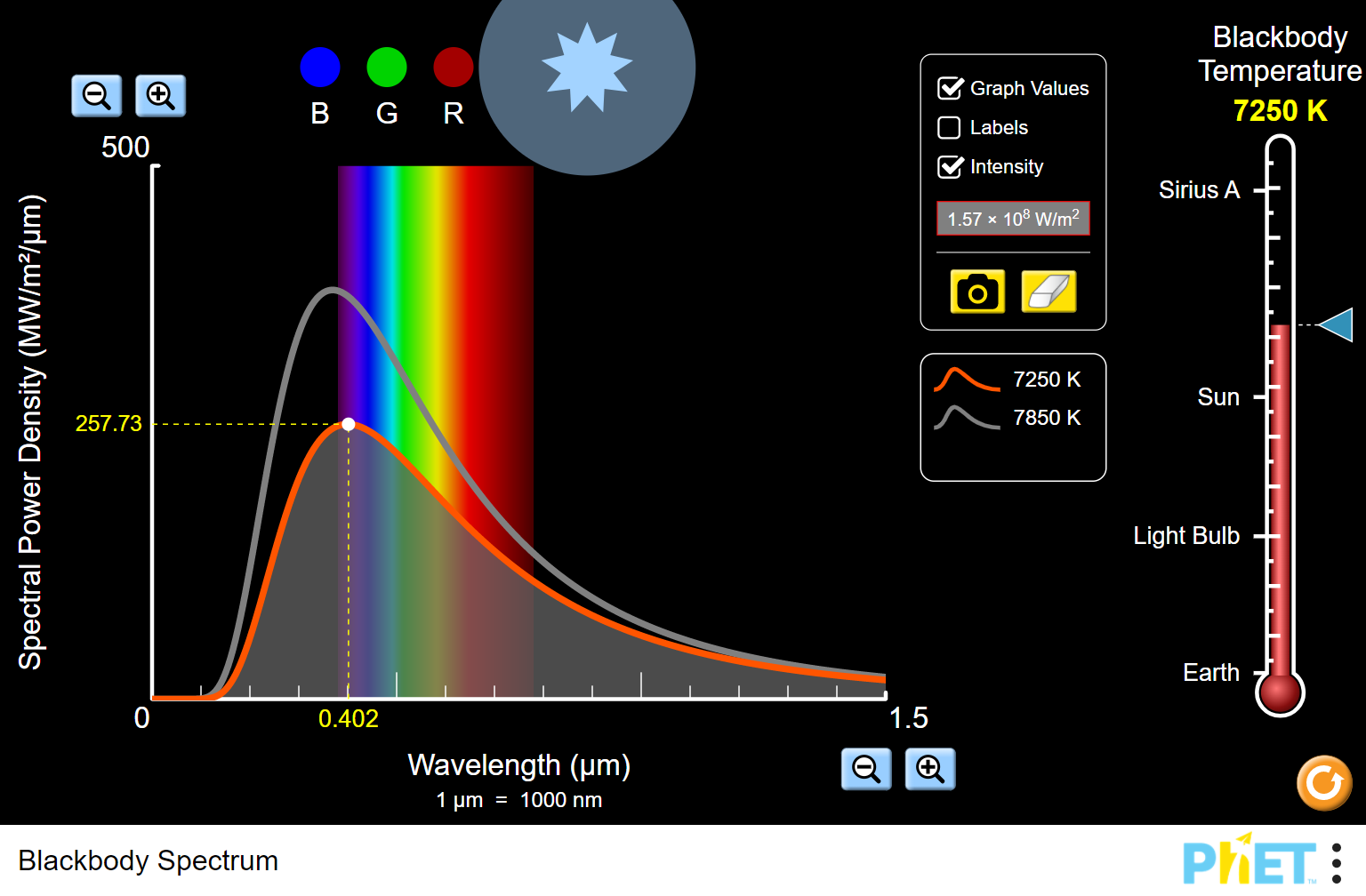
When a blackbody is at a uniform temperature, its emission has a characteristic frequency distribution that depends on the temperature. An object that absorbs ALL radiation falling on it, at all wavelengths, is called a blackbody. Conversely, all normal matter absorbs electromagnetic radiation to some degree. Understand the Rayleigh-Jeans Law and how it fails to properly model black-body radiationĪll normal matter at temperatures above absolute zero emits electromagnetic radiation, which represents a conversion of a body's internal thermal energy into electromagnetic energy, and is therefore called thermal radiation.Apply Wien’s Displacement Law to estimate the peak wavelength (or frequency) of the output from a black body radiator.Apply Stefan-Boltzmann’s Law to estimate total light output from a radiator.One experimental phenomenon that could not be adequately explained by classical physics was blackbody radiation. Integral Representation of the Distribution Not all radiators are blackbody radiators.


 0 kommentar(er)
0 kommentar(er)
